Understanding Sodium Channelopathies — Bridging mechanisms to new therapies
Imaging voltage-changes in cardiomyocytes differentiated from patient-derived iPSCs using Archon1 voltage-sensor
Voltage-gated sodium (NaV) channels are responsible for brisk spatial propagation of action potentials in cardiac myocytes and in neurons. Mutations in Na channels cause a wide-range of life-threatening diseases including arrhythmias, neurological and neurodevelopmental disorders - collectively knowns as sodium channelopathies . We study how Na channelopathic mutations disrupt channel structure, function, and regulation with the long-term goal of devising new mechanism-inspired therapies.
Cardiac Arrhythmias - In the heart, NaV1.5 channel dysfunction is associated with many pathologies, including congenital arrhythmias that stem from mutations and acquired arrhythmias in heart failure. Despite their distinct etiologies, a common mechanism is an enhanced late sodium current that results from incomplete inactivation of the channel. Although diminutive, this sustained influx of sodium ions prolongs the action potential and disrupts ion homeostasis. Thus, selective inhibitors of late sodium current are highly sought after as potential antiarrhythmics. In the lab, we are harnessing the power of nature to develop next-generation therapeutics using patient-derived iPSCs cardiomyocytes and mouse models. We engineered a peptide inhibitor named ‘FixR’ (FHF inhibiting x region) that selectively inhibits late sodium current with higher efficacy than the clinically-used inhibitor ranolazine.
Prototype of a late Na current inhibitor FixR
Epilepsy & Autism — Beyond the heart, mutations in the neuronal sodium channels (Na V 1.1/1.2/1.6) underlie epilepsy and seizure disorders as well as autism. We are interested in studying whether neuronal Na channelopathies, like their cardiac counterparts, also involve aberrant regulation by CaM, FGF12/13 and other cytosolic regulatory proteins. ( Potential rotation project ). If a unified mechanistic framework were substantiated, it would raise the possibility of developing common therapies, an exciting possibility.
Probing Calcium Channel regulation by Auxiliary Regulatory Proteins
Stac proteins inhibits Ca-dependent inactivation of L-type Ca channels
Voltage-gated calcium (CaV) channels serve as vital conduits for Ca2+ entry responsible for orchestrating diverse physiological processes including muscle contraction, vesicle secretion, and gene transcription. These channels are exquisitely modulated by auxiliary subunits and a vast array of regulatory proteins that intervene in several functions, like channel inactivation and adrenergic regulation. Mutations in any of these proteins may lead to structural and arrhythmic diseases of the heart. Still, several molecular mechanisms underlying regulation of CaV channels are not fully established. Using novel and state-of-the-art techniques, we probe interactions between potential regulatory proteins and CaV channels, as well as their effect on channel electrophysiology.
Stac Proteins - The neuron and muscle specific adaptor protein, stac, has emerged as important regulator of calcium channels essential for supporting excitation-contraction coupling in skeletal muscle and in tuning inactivation of L-type calcium channels in the brain. We are studying biophysical and physiological mechanisms underlying stac regulation of neuronal calcium channels.
Leucine Rich Repeat Containing protein 10 - A cardiac-specific protein involved in embryonic heart development, Lrrc10 has emerged as a key enhancer of calcium currents in both human and animal models. Mutations in LRRC10 have been associated with dilated cardiomyopathy, Brugada syndrome, and sudden unexplained nocturnal death syndrome. Our lab actively studies the biophysical mechanisms behind LRRC10 regulation of ion channels in both physiology and disease.
Developing New Tools to Study Ion Channel Assemblies and Function
Conceptual framework of FRET 2-hybrid assay for probing the stoichiometry and binding of macromolecular complexes in live cells.
FRET-based approaches for studying ion channel interaction and stoichiometry - FRET is a powerful methodology that is adept at quantifying protein-protein interactions. The interacting partners are tagged with appropriate donor and acceptor fluorescent proteins, and if they are in close proximity, photoexcitation of the donor results in fluorescence emission from the acceptor. The stoichiometry of the protein interactions and their relative binding affinities can be deduced by quantifying both the FRET efficiency and the total number of donors and acceptors in a given cell. In the lab, we try to expand this powerful technique into different biological system to dissect dynamic changes in ion channel interactions. We recently implemented FRET assays to a flow cytometer, which comes with high-throughput data acquisition, enhanced accessibility, and robust analysis.
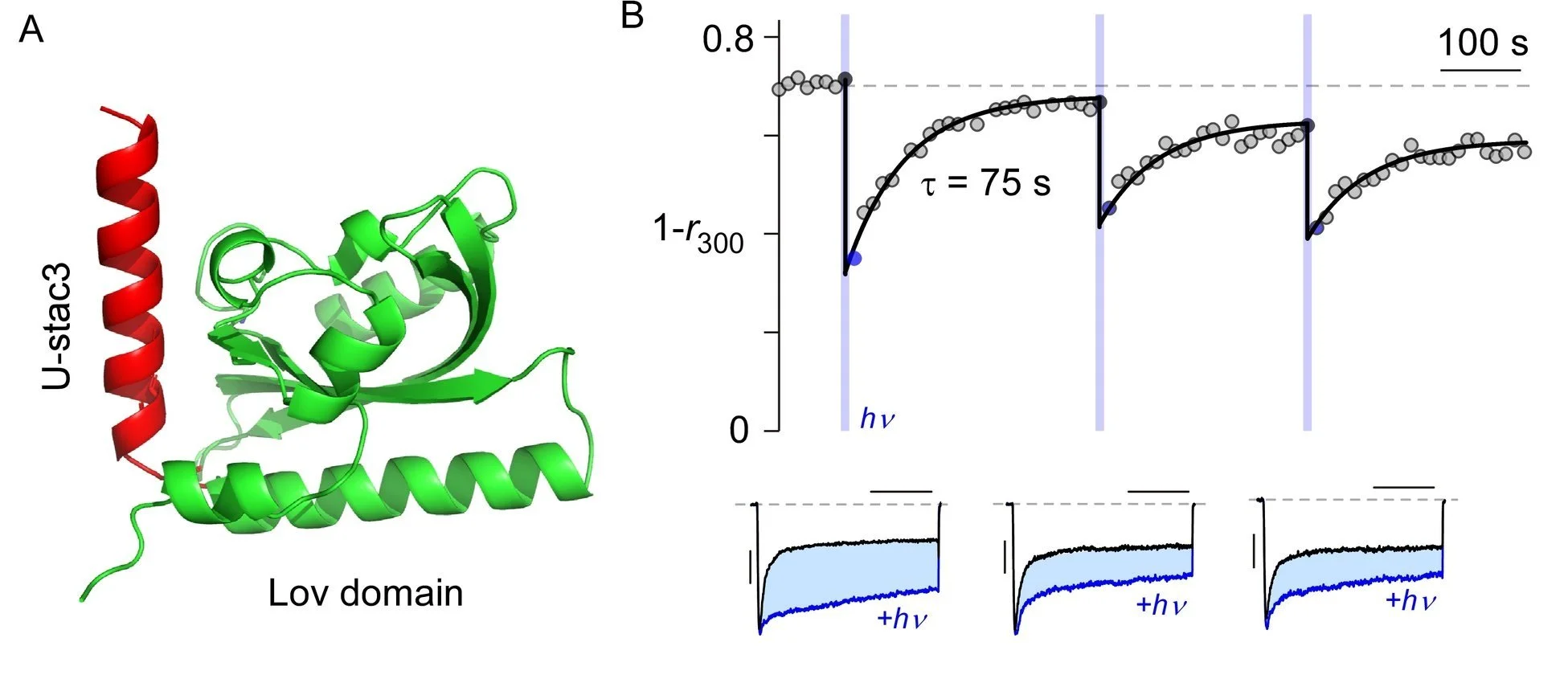
An optogenetic tool permits control of calcium channel kinetics
Devising Optogenetic Ca channel modulators - Optogenetic permit studies of excitable tissue through genetically-encodable and light-programmable actuators that acutely modify channel function. In the lab, we seek to engineer a light-sensitive AsLov2 domain from Avena sativa phototropin1 to photo-uncage a ‘natural’ calcium channel modulator. Specifically, AsLov2 contains a flavin mononucleotide (FMN) binding Lov domain that also associates with a helical Jα-segment in the dark. With blue light, a conformational change releases the Jα-helix, and switches the accessibility of an attached moiety. In darkness, this conformational change reverses and the Jα-helix rebinds the Lov2 domain. This allows unprecedented control of calcium channel kinetics in living cells.